Martian Greenhouse: A Micro-Ecosystem – Student Investigation
Designed for: Grades K-8 For use by:
Students, Teachers.
Learning Styles: Independent (student resource), Distance Learning, Interactive (hands-on)
Resource Type: Document, Guide
Students will investigate the difficulties in building a stable ecosystem containing higher organisms, such as tomatoes or other green plants, in a relatively small space.
Some important concepts
An Ecosystem: An ecosystem is a community of living organisms interacting with each other and their non-living environment.
community + habitat = ecosystem
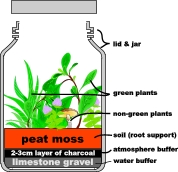
For this investigation we will provide a suitably prepared habitat within a sealed glass jar, into which we will place a small community of plants.
Dynamic Equilibrium
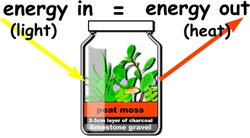

A Stable Ecosystem
A stable ecosystem is one in which, on average, a state of dynamic equilibrium exists.
A Simulated Martian Greenhouse
Step 1
To build a simulated Martian greenhouse, use a large empty wide-mouth jar (with a lid), like the commercial-size food containers such as Heinz ketchup and mustard used by restaurants.
Prepare the soil. The bottom layer should consist of a thin layer of gravel covered with a layer 2-3cm (1 inch) thick of charcoal. These layers act as soil and air buffers to help reduce large swings in the moisture content, and chemical composition, of the atmosphere in your simulated Martian greenhouse.
For root support the top layer of the soil should consist of a layer of peat moss. (For a more realistic Martian soil-simulation, a mixture of sterile sand and clay can be substituted, but its water retention properties are much less than that of peat moss).
Finally, plant an assortment of small green plants; the tomato seedlings from this project would be excellent candidates.
Step 2
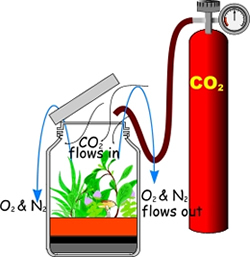
Note the alternative to this step using CO2 from a cylinder – see following.
Once the plants are installed you may wish to wait a few days to allow the plant roots to establish themselves in their new environment before proceeding to this step.
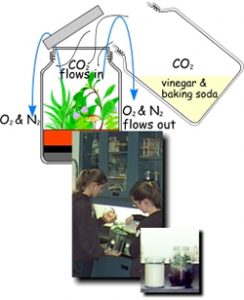
Prepare the rim of the jar with a light coat of vacuum grease or with a strip of Teflon plumber’s tape so that the lid can be installed immediately after the carbon dioxide has been poured into the jar.
To create a carbon dioxide atmosphere we will simply pour carbon dioxide (whose density is greater than that of air) into the jar. A simple source of carbon dioxide can be obtained by reacting a generous quantity of ordinary baking soda (sodium bicarbonate) with a generous quantity of cold vinegar (diluted acetic acid) in a very large container. Allow the reaction to subside, then carefully pour the carbon dioxide (which is denser than air) into the greenhouse.
Alternative to Step 2
Step 3
Before screwing down the lid, use a pair of tongs to insert a hot (120°C or 250oF ) bar of charcoal (which has been oven heated for at least one hour) into the jar. SEAL IMMEDIATELY!
Oven heating the bar of charcoal drives moisture and gases out of the bar. As the bar cools it will absorb an enormous quantity of carbon dioxide and will significantly reduce the gas pressure within the jar.
Step 4
The thin layer of vacuum grease (or Teflon ribbon) provides an airtight seal which preserves the low gas pressure within the jar, simulating a low pressure carbon dioxide Martian greenhouse atmosphere.
CAUTION: A sealed glass container should always be handled carefully.
Your simulated Martian greenhouse begins with a slight negative pressure of mostly CO2 but the pressure can drop dramatically because of carbon dioxide’s very high solubility in water.
Water on the other hand evaporates very rapidly under low pressure conditions. If your jar is left in a sunny or hot environment the pressure inside can rise well above normal atmospheric pressure, resulting is an exploding jar!
Always wear eye protection and gloves when handling your micro-ecosystem.
Tips for a successful Martian greenhouse
- Since your greenhouse is on the Earth, solar irradiation is quite high, therefore it is best not to expose your greenhouse to direct sunlight for more than 5 or 10 minutes per day.
- Stand or lay a small thermometer (the kind you see for attaching to coat zippers) in your ecosystem where it can be easily seen.
- Place a small piece of Litmus paper in your ecosystem so that you can monitor changes in the acid-base level of moisture within your ecosystem.
- A small amount (approximately 10-20 grams) of steel wool, washed with alcohol and rinsed with clean fresh water (to remove grease) and then mixed with the soil will not only remove excess oxygen from your greenhouse (rendering the atmosphere in the jar more Mars-like), it will also add iron oxides to the soil (making the soil more Mars-like too.)
- Plants adapt well to most light and temperature conditions (within a reasonable range), but they do not adapt well to frequent changes in their environment. Keep your Martian greenhouse in the same location, well lighted, and at a fairly constant temperature.
- Try to avoid “standing” water in your greenhouse. Before you add the carbon dioxide atmosphere check that the soil is moist, but not saturated, and that the inside bottom of the jar is just wet enough that water does not run from side to side within the jar when it is tilted.
- A few drops of liquid indoor-plant fertilizer added to the jar, according the manufacturer’s directions, will help stabilize the plants in their new environment.
- If you use the vinegar and baking soda method to produce carbon dioxide you will notice that it produces a large amount of foam and mist. Be careful not to inadvertently allow any of the foam or mist to enter your greenhouse jar.

Experience has shown that in a sample of ten or twelve micro-ecosystems), some will survive only a few weeks, others will last a few months, and rarely, a few will survive more than a year. The challenge is to determine, if possible, the reasons for the failure of some and the success of others.
A class discussion on this topic may elicit many hypotheses explaining the failure/success of their greenhouse simulators. This provides an excellent opportunity to have students invoke the Scientific Method and to have them design further experiments to test their hypothesis.
Martian Greenhouse - Student Investigation This Resource is part of the
This Resource is part of the